Highlights of the meeting of the Committee for Medicinal Products for Human Use (CHMP) 24-27 June 2024
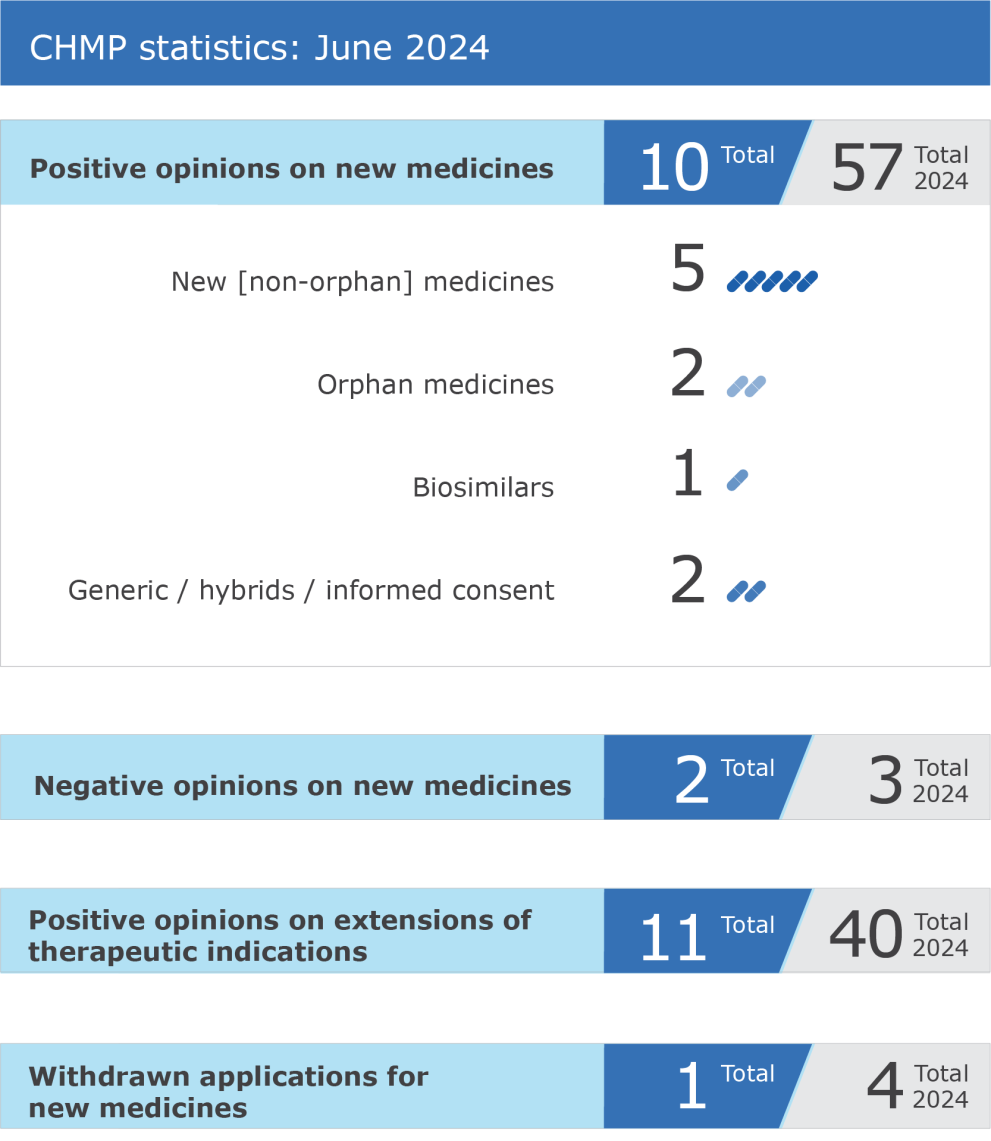
The EMA’s Committee for Medicinal Products for Human Use (CHMP) recommended ten medicines for approval at its June 2024 meeting. The Committee recommended that a marketing authorization be granted for Balversa (erdafitinib), for the treatment of adult patients with unresectable or metastatic urothelial carcinoma, a cancer of the bladder and urinary system. The CHMP has … Read more