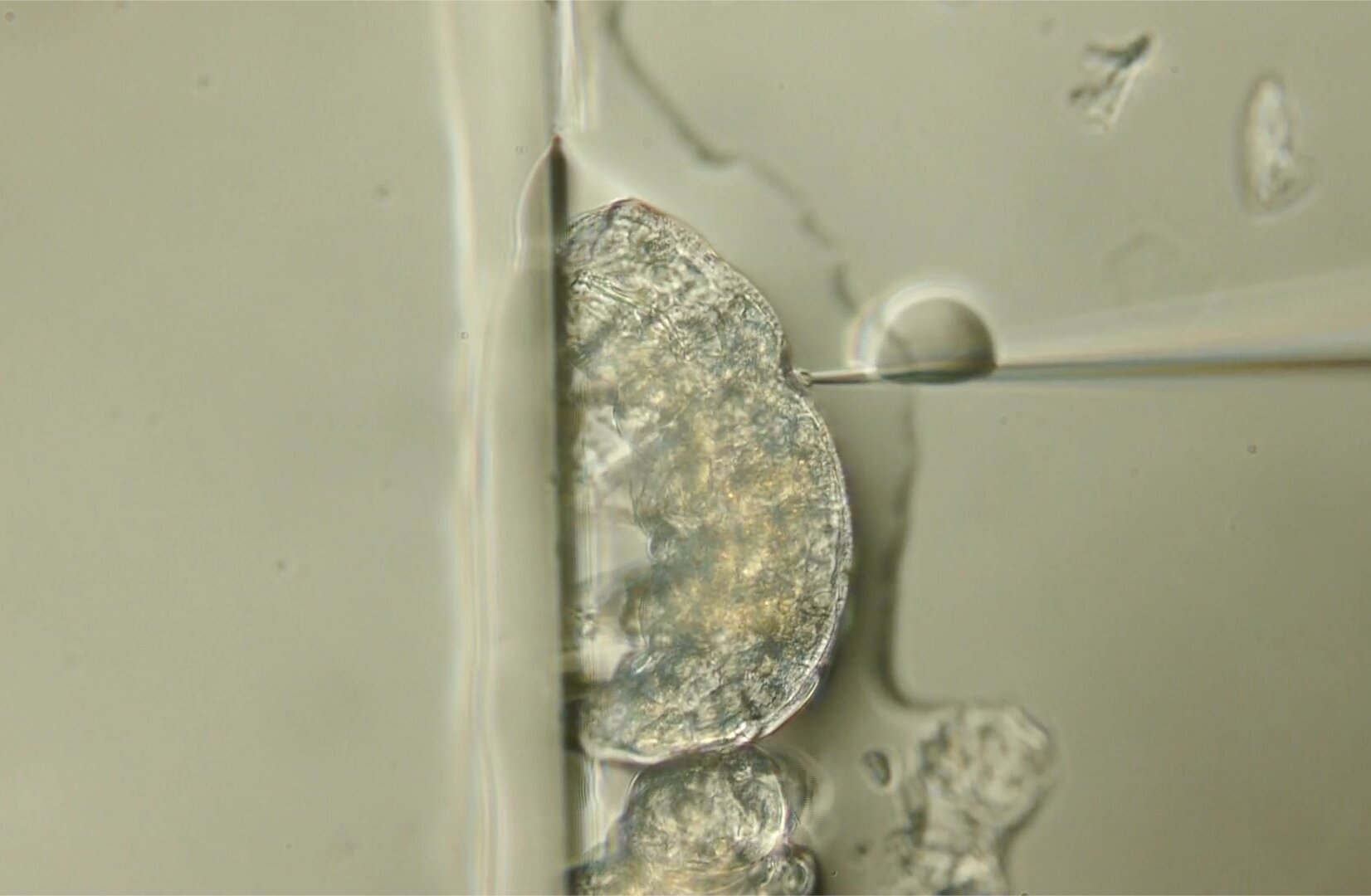
A tardigrade is given a dose of CRISPR tools to change one of its genes, and those of the eggs it will produce. Credit: 2024 Tokiko Saigo et al.
× close to
A tardigrade is given a dose of CRISPR tools to change one of its genes, and those of the eggs it will produce. Credit: 2024 Tokiko Saigo et al.
Some species of tardigrades are highly and unusually resilient to various extreme conditions that are fatal to most other life forms. The genetic basis for these exceptional abilities remains elusive.
For the first time, researchers from the University of Tokyo have successfully edited genes using the CRISPR technique in a highly resilient tardigrade species that was previously impossible to study with genome editing tools. The work has been published in PLoS genetics.
The successful delivery of CRISPR to an asexual tardigrade species directly produces gene-edited offspring. Designing and editing specific tardigrade genes allows researchers to investigate which ones are responsible for tardigrade resilience and how such resilience might work.
If you’ve heard about tardigrades, then you’ve undoubtedly heard of their unusual abilities to survive things like extreme heat, cold, drought, and even the vacuum of space, which several members of the species possess. So they naturally attract researchers who are eager to investigate these novelties, not only out of curiosity, but also to see what applications might one day be possible if we learn their secrets.
“To understand the superpowers of tardigrades, we must first understand how their genes function,” says Associate Professor Takekazu Kunieda from the Department of Biological Sciences.
“My team and I have developed a method to edit genes – by adding, deleting or overwriting them – as you would with computer data, in a highly tolerant species of tardigrade, Ramazzottius varieornatus. This now allows researchers to identify genetic traits of study tardigrade as they can be more established laboratory animals, such as fruit flies or nematodes.”
The team used a recently developed technique called direct parental CRISPR (DIPA-CRISPR), based on the now famous CRISPR gene editing technique, which can serve as a genetic scalpel to cut and modify specific genes more efficiently than ever before . DIPA-CRISPR has the advantage of being able to influence the genome of the offspring of a target organism and has previously been shown to work on insects, but this is the first time it has been used on non-insect organisms, including tardigrades.
Ramazzottius varieornatus is an all-female species that reproduces asexually, and almost all offspring were found to have two identical copies of the same edited code, unlike other animals, making it an ideal candidate for DIPA-CRISPR.
“We just had to inject CRISPR tools programmed to target specific genes for deletion in a parent’s body to obtain modified offspring, known as ‘knockout’ editing,” said Koyuki Kondo, project researcher at the time the research (currently an assistant professor in the Department of Life Science, Chiba Institute of Technology).
“We could also obtain gene-modified offspring by injecting additional DNA fragments that we want to include; this is called ‘knock-in’ editing. The availability of knock-in editing allows researchers to precisely edit tardigrade genomes, thus, for example, they control the way individual genes are expressed, or exhibit the functions of genes.”
The most important resilience trait of this species is their ability to survive extreme dehydration for long periods. It was previously shown that this is partly due to a special type of gel protein in their cells. And this property is interesting because it has also been applied to human cells.
Kunieda and other tardigrade researchers think it’s worth investigating whether something like an entire human organ could one day be successfully dehydrated and rehydrated without degradation. If possible, it could revolutionize the way organs are donated, transported and used in operations to save lives.
“I understand that some people are concerned about gene editing, but we conducted the gene editing experiments under well-controlled conditions and secured the edited organisms in a closed compartment,” Kunieda said.
“CRISPR could be an incredible tool to understand life and help with useful applications that could have a positive impact on the world. Not only do tardigrades give us a glimpse of what medical advances are possible, but their range of remarkable properties means they had an incredible evolutionary story, one we hope to tell when we compare their genomes to closely related creatures using our new DIPA-CRIPSR-based technique.”
More information:
One-step generation of homozygous knockout/knock-in individuals in an extremotolerant parthenogenetic tardigrade using DIPA-CRISPR, PLoS genetics (2024). DOI: 10.1371/journal.pgen.1011298
Magazine information:
PLoS genetics