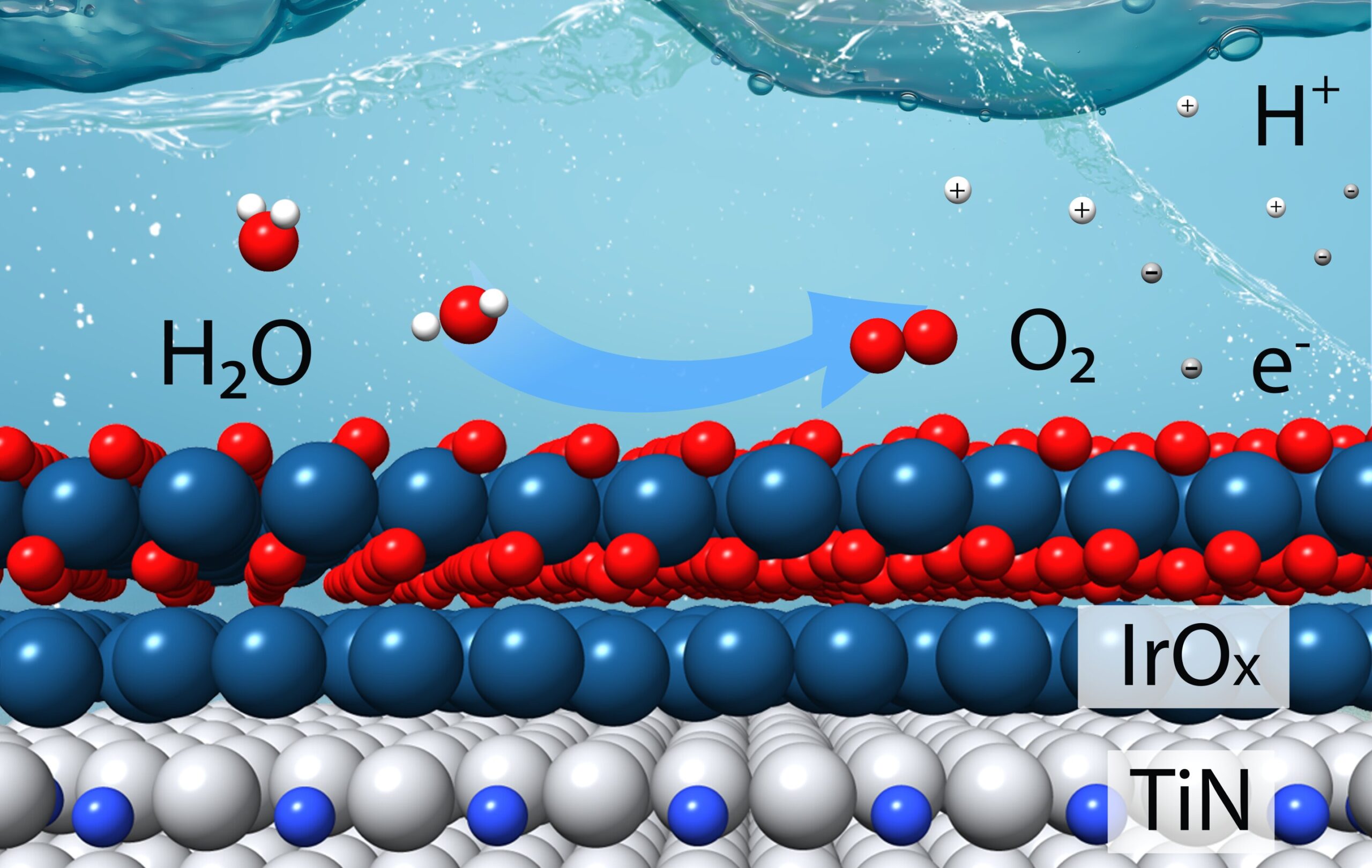
This diagram shows how a catalyst consisting of a few layers of iridium oxide (IrOX) over a titanium nitride (TiN) carrier can efficiently store oxygen (O2), hydrogen ions (H+), and electrons (e.g–) from water molecules (H2O) in an acidic electrolyte. This ‘oxygen evolution reaction’ is the more challenging of the two reactions required to split water to produce hydrogen gas (H2). Credit: Tianyou Mou/Brookhaven National Laboratory
Hydrogen (H2) is a promising fuel for reducing greenhouse gases, especially if produced by using renewable energy to split water molecules (H2O). But as simple as it seems to split water into hydrogen and oxygen, the chemistry is complex.
Two separate simultaneous electrochemical reactions each require catalysts, chemical “deal makers” that help break and re-form chemical bonds. Now scientists from the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and Columbia University say they have developed a new efficient catalyst for the more challenging part: the oxygen evolution reaction.
As described in an article just published in the Journal of the American Chemical Societythe catalyst was designed “from the bottom up” based on theoretical calculations aimed at minimizing the amount of iridium, an expensive metal used as a catalytic material, and maximizing the catalyst’s stability under acidic conditions.
When the team created models of the catalyst and tested them in the laboratory, the results confirmed the predictions. The scientists then made a powder form of the catalyst, as used in industrial applications, and showed that it can efficiently produce hydrogen in a water-splitting electrolyzer.
“In this field test, our catalyst is approximately four times better than the most modern commercially available iridium catalyst,” said Jingguang Chen, a chemical engineer at Columbia University with a joint appointment in the Department of Chemistry at Brookhaven. Lab that conducted the research. In other words, the new catalyst requires four times less iridium to produce hydrogen at the same rate as the commercial variant, or produces hydrogen four times faster for the same amount of iridium.
Brookhaven Lab theoretical chemist Ping Liu, who led the calculations underlying the catalyst design, said: “This study shows how you can go from a theory-driven understanding of what’s happening at the atomic level to designing a catalyst for practical use gives us a better understanding of how this catalyst works and brings us closer to real-world application.”
The remaining challenge is scaling up production.
“We only make milligrams of catalyst per batch,” Chen said. “If you want to make megatons of green hydrogen, you need kilos or tons of catalyst. We cannot yet do that on that large scale.”
Reduce iridium
Iridium is the catalyst of choice for the oxygen evolution reaction, which takes place at the anode of an electrolyzer. It supplies the electrically charged active sites that separate tightly bound hydrogen ions (H+) from oxygen (O). In addition to liberating the H+ ions – which contribute to the highly acidic reaction conditions – the reaction produces oxygen gas (O2) and electrons. Those electrons are needed for the second, less challenging “hydrogen evolution” reaction: linking hydrogen ions to form hydrogen gas at the electrolyzer’s cathode.
“Iridium is currently one of the few stable elements for the oxygen evolution reaction in acid,” Chen said. That’s “unfortunate,” he noted, because “iridium is even rarer and more expensive than platinum.”
Hence the motivation to reduce the amount of iridium.
“In industrial catalysts made of nanoscale particles, only atoms on the surface participate in the reaction,” Chen said. “That means most of the iridium on the inside of the particle is wasted.”
Perhaps instead of using a particle made entirely of iridium, a catalyst could be made from a cheaper material with only iridium on the surface, the team reasoned.
The team had explored the use of elements commonly found on Earth, such as titanium. They found that combining titanium with nitrogen provided enough stability for these “titanium nitrides” to survive acidic reaction conditions. Perhaps titanium nitride could serve as the core of iridium-coated catalytic particles.
But how much iridium should be put on top? This is where the theoretical calculations come in.
Calculation of an ideal structure
“We used ‘density functional theory’ calculations to model how different overlayers of iridium on titanium nitride would affect the catalyst’s stability and activity under acidic oxygen evolution reaction conditions,” Liu said. She and her team used computing resources at Brookhaven Lab’s Center for Functional Nanomaterials (CFN) and at DOE’s Lawrence Berkeley National Laboratory’s National Energy Research Scientific Computing Center (NERSC) to run the simulations.
The calculations predicted that one layer of iridium would not be sufficient to drive the oxygen evolution reaction, but that two or three layers would improve both performance and catalytic stability.
“These were kind of pre-screening experiments,” Liu said. “We then transferred these screening results to the experimental team to create real catalysts and evaluate their catalytic activity.”
Validating the predictions
First, the team created thin films in which they could create carefully controlled layers that closely resembled the surfaces used in the theoretical modeling calculations. They also created powder samples composed of tiny nanoscale particles, the form the catalyst would take in industrial applications. They then studied the thin films (including the interfaces between the layers) and the nanoparticles using different techniques.
These include transmission electron microscopy at CFN and X-ray spectroscopy studies at the Quick X-ray Absorption and Scattering (QAS) beamline of the National Synchrotron Light Source II (NSLS-II), a source of bright X-rays for deciphering samples. chemical and physical properties.
“Our hypothesis was that if the iridium bonded to the titanium nitride, this bond would stabilize the iridium and improve the reaction,” Chen said.
The characterization studies confirmed the predictions.
“The synchrotron studies revealed the oxidation states and local coordination environment of the iridium and titanium atoms under reaction conditions,” Chen said. “They confirmed that iridium and titanium have a strong interaction.”
“Elemental mapping of the nanoparticles at CFN confirmed the particle sizes and compositions, including the presence of iridium oxides on the surface over titanium nitride supports,” he added.
Liu emphasized that the characterization studies informed the scientists’ understanding of the catalyst.
“We found that the interaction between iridium and titanium is useful not only for the stability of the catalyst, but also for fine-tuning its activity,” she said. “The charges change the chemistry in a way that improves the reaction.”
Specifically, charges transferred from titanium to the iridium surface change the electronic structure of the iridium active sites to optimize the binding of reaction intermediates, she explained.
“If you go from one to three layers of iridium, you significantly increase the charge transfer from the nitride to the top iridium,” Liu noted. But the difference between two and three layers was not very big. Two layers may be sufficient to enable high stability, activity and low cost.
To make this catalyst ready for real-world use, the scientists pointed out that in addition to tackling the challenge of scaling up production, improvements can also be made to optimize the consistency of the powders.
“When we make thin films, we can control the layers, but with powder synthesis we don’t have that kind of control,” Chen said. “Our powder particles do not have a continuous iridium shell around them, but this study provides guidance that industrial chemists could use to create true core-shell structures with a uniform thin layer of iridium,” he said.
Such catalysts could help reduce the cost of water splitting and move scientists closer to producing large quantities of green hydrogen.
More information:
Xue Han et al., Theoretical Prediction and Experimental Verification of IrOx Supported on Titanium Nitride for Acid Oxygen Evolution Reaction, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c02936
Provided by Brookhaven National Laboratory
Quote: Scientists create and test efficient water-splitting catalyst predicted by theory (2024, June 11), retrieved June 12, 2024 from https://phys.org/news/2024-06-scientists-efficient-catalyst-theory.html
This document is copyrighted. Except for fair dealing purposes for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.