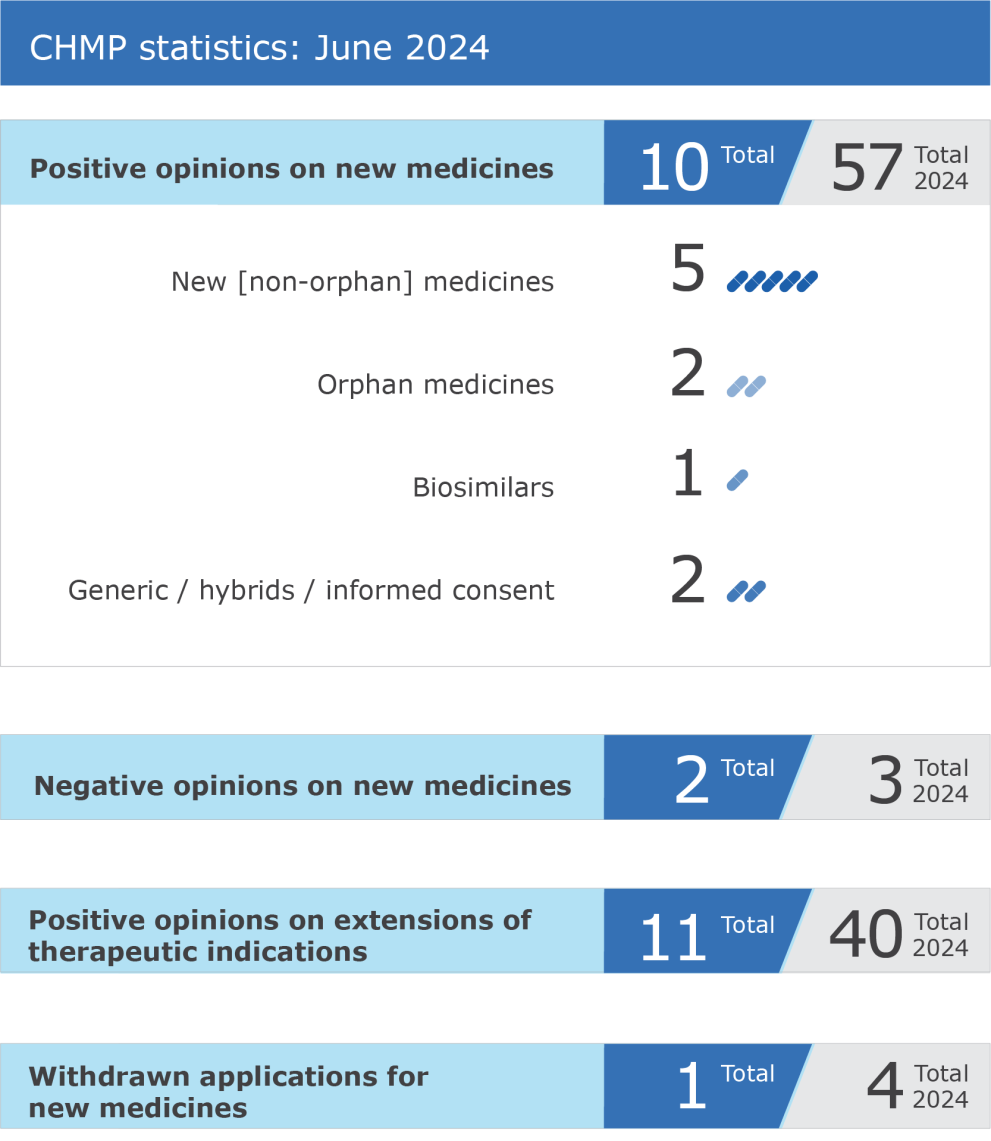
The EMA’s Committee for Medicinal Products for Human Use (CHMP) recommended ten medicines for approval at its June 2024 meeting.
The Committee recommended that a marketing authorization be granted for Balversa (erdafitinib), for the treatment of adult patients with unresectable or metastatic urothelial carcinoma, a cancer of the bladder and urinary system.
The CHMP has issued a positive opinion for Eurneffy (epinephrine), the first emergency treatment for allergic reactions that is administered as a nasal spray, not as an injection. See more details in the news announcement in the grid below.
mResvia (Respiratory syncytial virus (RSV) mRNA vaccine) received a positive opinion from the CHMP for the prevention in adults aged 60 years and over of lower respiratory tract disease and acute respiratory illness caused by respiratory syncytial virus, a common respiratory virus that usually causes mild, cold-like causes symptoms, but can lead to serious consequences in the elderly. This is the first mRNA vaccine targeting a pathogen other than SARS-CoV-2 to receive a positive opinion from the CHMP.
The Commission recommended granting a conditional marketing authorisation for Order* (odronextamab), to treat follicular lymphoma and diffuse large B-cell lymphoma, two types of blood cancers that affect the immune system.
Piasky (crovalimab) has been given a positive opinion by the CHMP for the treatment of paroxysmal nocturnal haemoglobinuria, a rare genetic condition that causes the premature breakdown of red blood cells by the immune system and is potentially life-threatening.
The CHMP has given a positive opinion for Tauvid (flortaucipir (18F)), for positron emission tomography (PET) imaging of the brain in adult patients with cognitive impairment being evaluated for Alzheimer’s disease.
The CHMP recommended that marketing authorization be granted for Winrevair* (sotatercept), for the treatment of adult patients with pulmonary arterial hypertension, a rare, long-term, disabling and life-threatening condition in which patients have abnormally high blood pressure in the arteries of the lungs. This medicine was supported through the EMA’s Priority Medicines (PRIME) programme, which provides early and enhanced scientific and regulatory support for promising medicines that have the potential to address unmet medical needs. See more details in the news announcement in the grid below.
The Committee recommended that a marketing authorization be granted for Steqeyma (ustekinumab), a biosimilar medicine for the treatment of adult patients with moderately to severely active Crohn’s disease, plaque psoriasis, pediatric plaque psoriasis and psoriatic arthritis.
The committee has also issued positive opinions for two generic medicines:
- Enzalutamide Viatris (enzalutamide) for the treatment of prostate cancer.
- Nilotinib agreement (nilotinib) for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia.
Negative recommendations for two medications
The CHMP has recommended that the marketing authorisation for this medicine be refused. Masitinib AB Science* (masitinib), a medicine intended to treat amyotrophic lateral sclerosis, a rare disease of the nervous system that leads to loss of muscle function and paralysis, and Syfovre (pegcetacoplan), for the treatment of geographic atrophy secondary to age-related macular degeneration, a progressive macular disease of the retina that causes gradual loss of vision, mainly in the elderly.
For more information on these negative opinions, see the Q&A documents in the chart below.
Non-renewal of the conditional marketing authorisation
The Committee recommended the conditional marketing authorization Translarna* (ataluren), a medicine to treat patients with nonsense mutation Duchenne muscular dystrophy, a genetic disorder characterised by progressive loss of muscle mass. This CHMP opinion will now be forwarded to the European Commission for a final legally binding decision applicable in all European Union (EU) Member States.
For further information on the non-renewal of this conditional marketing authorisation, please see the Public Health Communication in the chart below.
Recommendations for extensions of therapeutic indications for 11 medicinal products
The committee recommended expanding the indication for 11 medicines already authorized in the EU: Betmiga, Beyfortus, Cresemba, Unknown*, Nonsense, Infanrixhexa, Lynparza, Pegasys, Tepkinly*, Vabysmo And Xalkori.
Withdrawal of the application
One application for an initial marketing authorization was withdrawn. Dabigatran etexilate Teva (dabigatran etexilate) was intended for the prevention of venous thromboembolic events.
A question and answer document regarding the withdrawal of Dabigatran Teva is available in the chart below.
Conclusion of references
The committee completed an evaluation of Havrix, a vaccine used to protect adults and children against infection with the hepatitis A virus, and recommended changes to the prescribing information to harmonize the way the medicine is used in the EU. A Q&A document is available in the grid below.
The CHMP has completed a review of Lorazepam Macurea medicine of the benzodiazepine class, following disagreement between EU Member States over a request to update the product information of the medicine to include the treatment of status epilepticus in adults, adolescents and children from one month of age. A question and answer document on this update is available in the grid below.
The EMA’s Human Medicines Committee has completed its investigation Ocaliva*a medicine used to treat adults with a rare liver disease known as primary biliary cholangitis, and recommended withdrawing the conditional marketing authorisation for the medicine because its benefits no longer outweigh its risks. For more information on the recommendation to withdraw this conditional marketing authorisation, see the public health message in the grid below.
Other updates
The CHMP gave a positive opinion to update the composition of the mRNA vaccine Comoros to target the new SARS-CoV-2 JN.1 variant of the virus that causes COVID-19. The revision of this vaccine is in line with the recommendation of the EMA Emergency Task Force to update the COVID-19 vaccines and focus them on the SARS-CoV-2 variant JN for the 2024/2025 vaccination campaign .1.
Agenda and minutes
The agenda of the June 2024 CHMP meeting will be published on the EMA website. The minutes of the meeting will be published in the coming weeks.
CHMP statistics
Key figures from the June 2024 CHMP meeting are shown in the graph below.
*This product was designated an orphan drug during its development. Orphan drug designation is assessed at the time of approval by the EMA’s Committee for Orphan Medicinal Products (COMP) to determine whether the information available to date allows the drug to be retained orphan drug status and granted 10 years of market exclusivity.