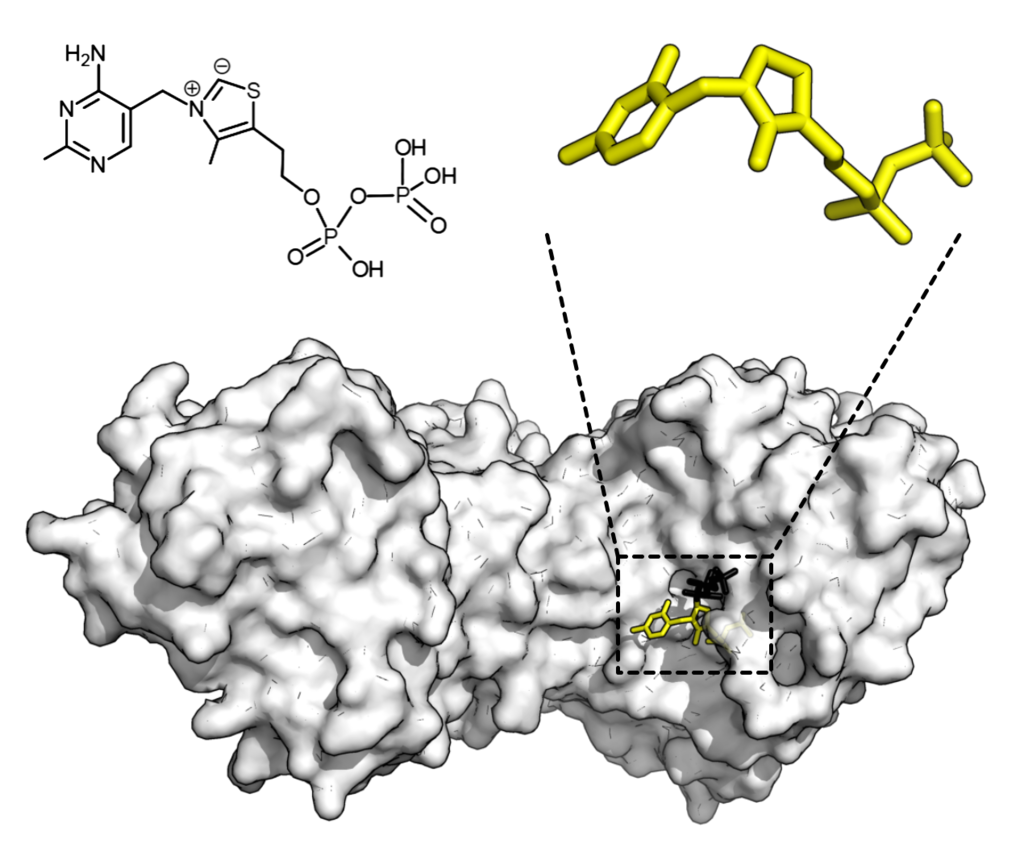
Chemical structure for thiamine pyrophosphate and protein structure of transketolase. Thiamine pyrophosphate cofactor in yellow and xylulose 5-phosphate substrate in black. Credit: Thomas Shafee/Wikipedia
× close to
Chemical structure for thiamine pyrophosphate and protein structure of transketolase. Thiamine pyrophosphate cofactor in yellow and xylulose 5-phosphate substrate in black. Credit: Thomas Shafee/Wikipedia
Scientists have developed a prototype of a new method for ‘rationally manipulating’ enzymes to achieve better performance. They have devised an algorithm that takes into account the evolutionary history of an enzyme to indicate where mutations can be introduced with a high probability of functional improvements.
Their work, published today in the magazine Nature communication– could have significant, far-reaching impacts on a range of industries, from food production to human health.
Enzymes play a central role in life and are crucial for the development of innovative medicines and tools to address society’s challenges. They have evolved over billions of years through changes in the amino acid sequence that underlies their 3D structure. Like beads on a string, each enzyme consists of a series of hundreds of amino acids that code for its 3D shape.
Because one of twenty amino acid beads is possible at each position, enormous sequence diversity is possible in nature. In forming their 3D shape, enzymes perform a specific function, such as digesting our dietary proteins, converting chemical energy into force in our muscles, and destroying bacteria or viruses that invade cells. If you change the order, you can disrupt the 3D shape, and that usually changes the functionality of the enzyme, sometimes rendering it completely ineffective.
Finding ways to improve the activity of enzymes would be enormously beneficial for many industrial applications and using modern tools in molecular biology it is simple and cost-efficient to effect changes in the amino acid sequences to enable improvements in their performance. However, randomly introducing just three or four changes into the sequence can lead to a dramatic loss of their activity.
Here, the scientists report a promising new strategy to rationally develop an enzyme called ‘beta-lactamase’. Instead of introducing random mutations in a scattergun approach, researchers at the Broad Institute and Harvard Medical School developed an algorithm that takes into account the enzyme’s evolutionary history.
“At the heart of this new algorithm is a scoring function that exploits thousands of beta-lactamase sequences from many different organisms. Instead of a few random changes, up to 84 mutations over a sequence of 280 were generated to improve functional performance,” said Dr . Amir Khan, associate professor at Trinity College Dublin’s School of Biochemistry and Immunology, one of the co-authors of the study.
“And remarkably, the newly designed enzymes had both improved activity and stability at higher temperatures.”
Eve Napier, a second-year Ph.D. student at Trinity College Dublin, determined the experimental 3D structure of a newly designed beta-lactamase, using a method called X-ray crystallography.
Her 3D map showed that despite changes in 30% of the amino acids, the enzyme had an identical structure to the wild-type beta-lactamase. It also revealed how coordinated changes in amino acids, introduced simultaneously, can efficiently stabilize the 3D structure – unlike individual changes that typically affect the enzyme structure.
Eve Napier said: “Overall, these studies show that proteins can be manipulated for enhanced activity through dramatic ‘jumps’ in new sequence space.
“The work has broad applications in industry, in processes that require enzymes for food production, enzymes that break down plastic and are relevant to human health and disease, so we’re quite excited about the future possibilities.”
More information:
Nature communication (2024). DOI: 10.1038/s41467-024-49119-x
Magazine information:
Nature communication