Credit: Cell host and microbe (2024). DOI: 10.1016/j.chom.2024.05.008
A team of UC Davis Health researchers has discovered that a commonly used anti-inflammatory drug, mesalamine, can replace the work of good bacteria in fighting the nasty fungus Candida albicans in the intestines.
C. albicans, or candida, is known to cause fungal infections. In some cases it develops into invasive candidiasis, a potentially fatal infection that occurs mainly in immunocompromised patients.
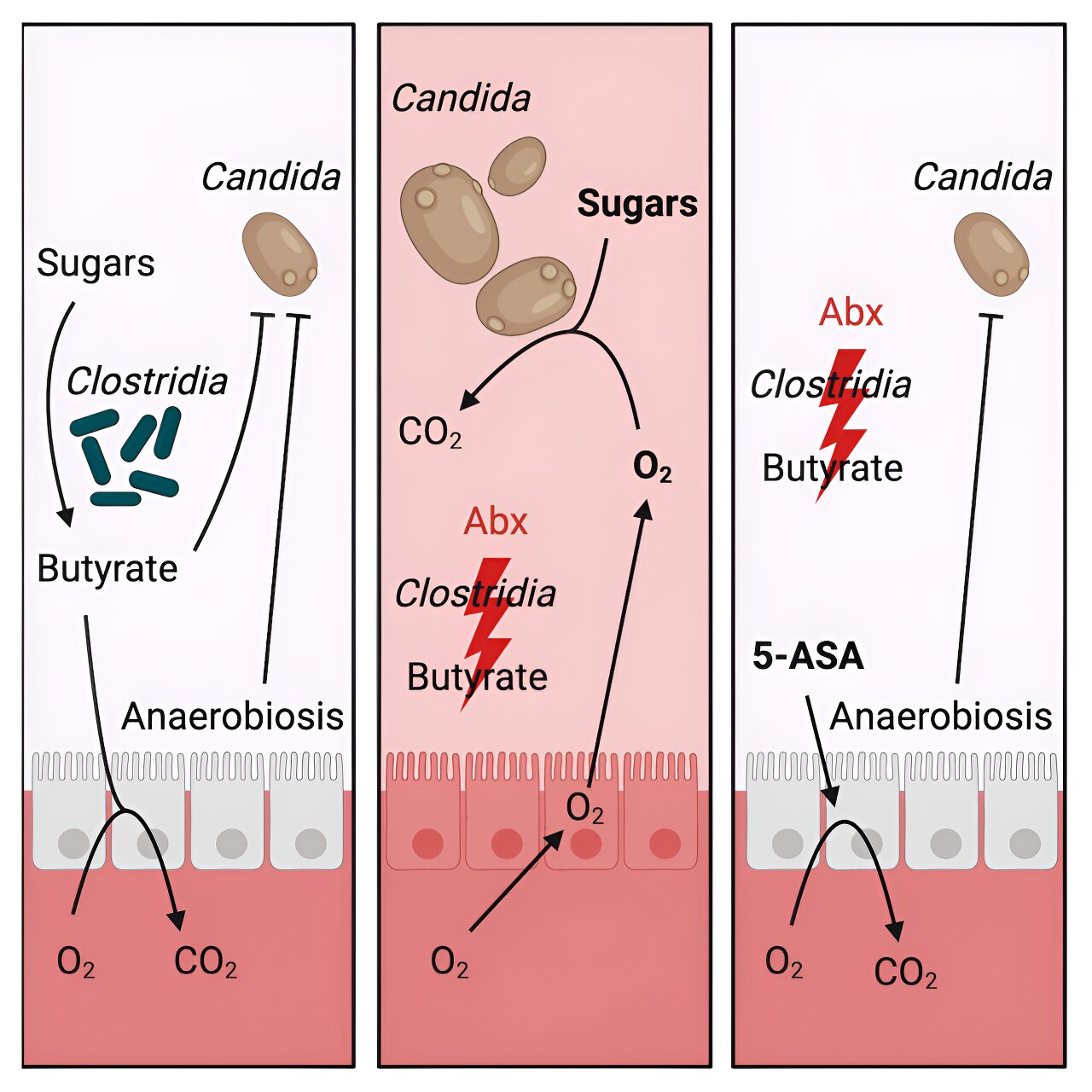
The researchers discovered that this fungus cannot grow without an oxygen supply. Their research in mice showed that the drug can maintain a low-oxygen environment (hypoxia) that prevents fungal blooms in the intestines.
Their study appears today in Cell host and microbe
Antibiotic use can lead to fungal blooms in the intestines
The team studied how C. albicans colonize the intestine. The fungus, best known for causing vaginal yeast infections, is usually treated with a topical or oral antifungal medication without serious side effects. It also lives harmlessly in the intestines of about 60% of people.
But if the body’s immunity wanes due to cancer or chemotherapy, the fungus can grow outside the colon and spread throughout the body. In such cases, the patient develops invasive candidiasis.
“Invasive candidiasis is a potentially fatal infection with a mortality rate of approximately 50%. That is even with the best available treatment,” explains Andreas Bäumler, lead author of the study. Bäumler is a distinguished professor in the Department of Medical Microbiology and Immunology.
Patients with leukemia and other blood cancers may need to take antibiotics. This use can cause an imbalance in the intestinal microbial community. It reduces Clostridia, a group of bacteria that promotes resistance to fungal colonization in the intestines. With fewer Clostridia, C. albicans grows and colonizes in the intestinal tract.
“A bloom of C. albicans in the intestines during antibiotic therapy is the most common cause of candidemia in people being treated for blood cancer,” explains Bäumler. Candidemia is the presence of fungi or yeasts in the blood.
Bäumler and his team wanted to understand the factors involved in antibiotic-induced colonization of C. albicans in the intestines.
Candida loves simple sugars and oxygen
They first colonized germ-free mice with Candida to see what the fungus needed to thrive. They realized that Candida really liked simple sugars, similar to those found in high-sugar diets. They then tested its growth in a petri dish. They placed Candida with simple sugars in an aerobic (with oxygen) environment, and the fungi started to flourish.
“A healthy intestine has little oxygen. That’s why we repeated the test in a hypoxia environment,” Bäumler said. The fungi did not grow despite the presence of sugars. This meant that oxygen is a necessary condition for the growth of Candida.
The role of probiotics in preventing fungal growth
The team conducted a series of experiments showing that antibiotic use reduced Clostridia in the intestines. Giving mice probiotics, such as Clostridia, prevented C. albicans from growing in their intestines. Still, probiotics can be killed by antibiotics and cancer therapy. For this reason, probiotics would not help patients with leukemia or other forms of blood cancer.
“Probiotics are often not safe in patients at highest risk for invasive candidiasis,” Bäumler said. “Finding a therapy that can function like probiotics but tolerate the impact of cancer treatment and antibiotics was important.”
Anti-inflammatory drugs as faux-biotics
The team investigated 5-aminosalicylic acid (5-ASA) as a safer way to control C. albicans in the intestines. 5-ASA, also called mesalamine, is an anti-inflammatory drug. It is used to treat inflammatory bowel diseases (IBD), such as Crohn’s disease and ulcerative colitis.
The team tested 5-ASA in mice treated with antibiotics. They found that the drug could replace the work of probiotics by preventing oxygen from entering the colon and C. albicans from expanding in the intestines.
“Limiting oxygen in the intestines by replacing the function of good bacteria could be a strategy to reduce invasive candidiasis,” Bäumler said. “Our study opens up a completely new treatment option for deadly fungal infections, especially for patients with cancer. After all, fungi cannot become resistant to hypoxia.”
The team proposed the term ‘faux-biotics’ to refer to products, such as 5-ASA, that mimic the function of probiotics such as Clostridia.
The study’s first co-authors are Hannah Savage, Derek Bays and Connor Tiffany. The other co-authors are Mariela Gonzalez, Eli Bejarano, Thaynara Carvalho, Zheng Luo, Hugo Masson, Henry Nguyen, Renato Santos, Krystle Reagan and George Thompson of UC Davis.
More information:
Hannah P. Savage et al., Epithelial hypoxia maintains colonization resistance to Candida albicans, Cell host and microbe (2024). DOI: 10.1016/j.chom.2024.05.008. www.cell.com/cell-host-microbe … 1931-3128(24)00180-X
Quote: An anti-inflammatory substance inhibits the spread of fungi that cause serious blood infections (2024, June 4) retrieved on June 5, 2024 from https://medicalxpress.com/news/2024-06-anti-inflammatory-curbs-fungi-blood.html
This document is copyrighted. Except for fair dealing purposes for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.