Dimensionally controlled assemblies of NiII norcorroles based on the interactions between anti-aromatic systems. Credit: Chemical Science (2024). DOI: 10.1039/D4SC01633E
× close to
Dimensionally controlled assemblies of NiII norcorroles based on the interactions between anti-aromatic systems. Credit: Chemical Science (2024). DOI: 10.1039/D4SC01633E
In organic chemistry, π-stacking systems are supramolecular structures that arise as a result of the dispersion force, a type of intermolecular noncovalent interaction. They are a common phenomenon in nature. The stabilized structure of DNA is a very prominent example of a π-stacking system, as is the arrangement of amino acids in certain proteins.
Interestingly, π-stacking can be used in the design of materials with useful electronic and optical properties. These include organic semiconductors of various types, as well as conjugated polymers for sensing and biomedical applications.
To date, a large portion of technologically relevant π-stacking systems have been limited to aromatic compounds, which have inherent π-electron clouds. On the other hand, antiaromatic compounds, although promising candidates for the development of electrical conductors, have rarely been reported as building units of π-stacking systems.
Surprisingly, in a recent study, a research team led by Professor Hiromitsu Maeda from Ritsumeikan University, Japan, reported a new anti-aromatic π-stacking system that enabled the formation of a highly conductive liquid crystal.
Their findings were published in the journal on April 16, 2024 Chemical Science. The article is co-authored by Prof. Go Watanabe of Kitasato University, Prof. Shu Seki of Kyoto University and Prof. Hiroshi Shinokubo of Nagoya University.
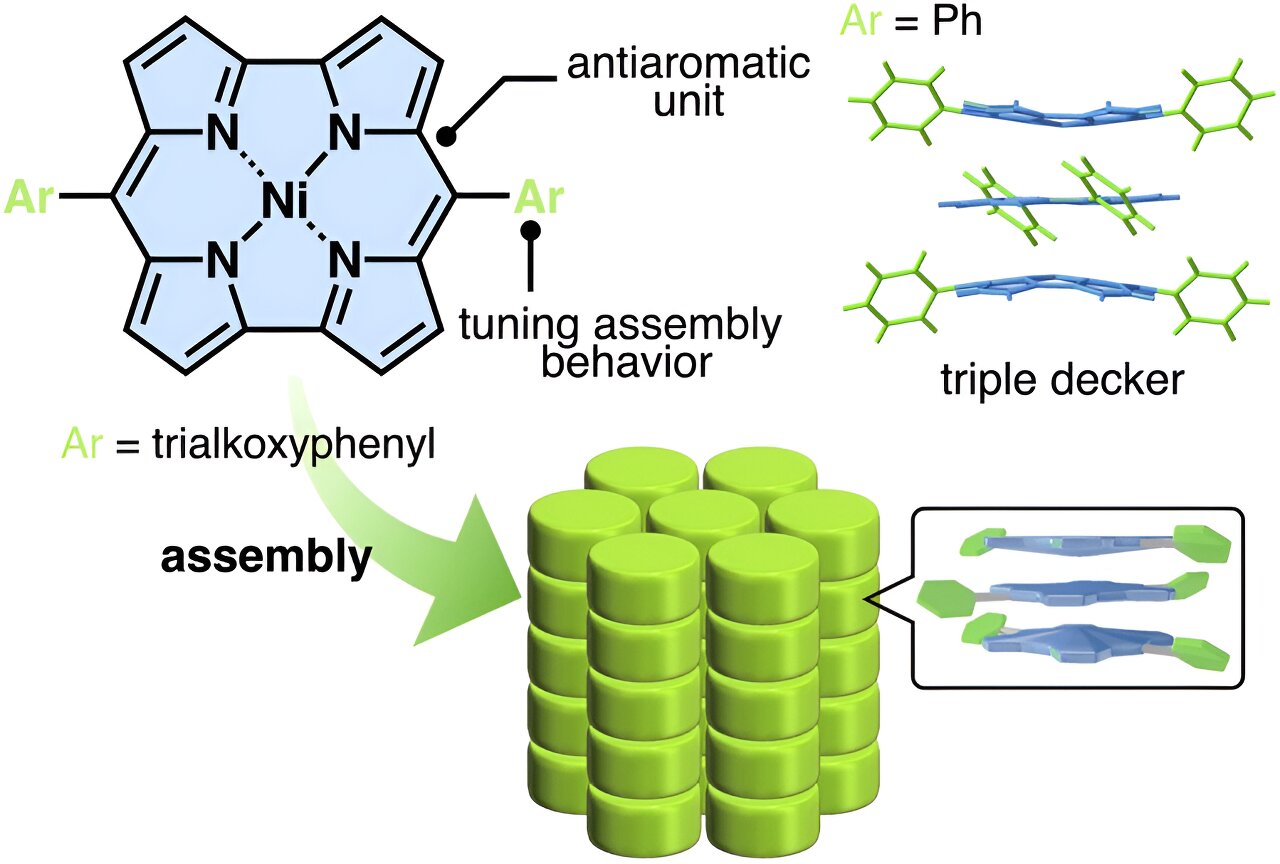
The reported compounds in question are NiII-coordinated norcorroles with modified aryl groups as side chains. Previously, achieving π-stacking in similar norcorroles failed because hydrogen-bonding interactions between the side chains opposed the face-to-face stacking of the planar antiaromatic units. This time, however, the research team had an ingenious idea.
“We hypothesized that the introduction of lateral interaction groups with less directionality would improve stacking between norcorrole units,” explains Prof. Maeda. “We thus attempted the simple introduction of aliphatic chains, which induce van der Waals interactions. These interactions can be effective for modulating the stacking structure of a material.”
As shown by several experiments and molecular dynamics simulations, the proposed strategy worked as intended. The norcorrole units formed columnar structures by stacking arrangements known as “triple-decker”. In these arrangements, a flat molecule is sandwiched between two slightly cup-shaped molecules.
Using the proposed molecular design, the researchers then synthesized liquid crystals. Thanks to the triple stacking, a liquid crystal showed remarkable electrical conductivity and thermotropity; that is, an order parameter that depends on temperature.
“The control of molecular interactions based on molecular design and synthesis, as demonstrated in our study, will be crucial for future applications,” says Prof. Maeda. “Properties such as high electrical conductivity in liquid crystals can be used for the fabrication of electronic devices. Furthermore, stimuli-responsive behavior in soft materials can be used to modulate relevant properties, such as photoluminescence, based on pressure and temperature.”
Taken together, the findings of this study reveal a promising strategy for designing new compounds based on molecular assemblies of antiaromatic moieties. With any luck, this will open new avenues for materials design, ultimately leading to better organic electronics, optoelectronics and sensor devices.
More information:
Soh Ishikawa et al, Norcorroles as anti-aromatic π-electronic systems forming dimensionally controlled assemblies, Chemical Science (2024). DOI: 10.1039/D4SC01633E
Magazine information:
Chemical Science